Cr2 Co3 3 Compound Name
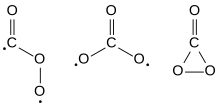 | |
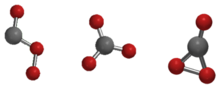 The Cs, D3h, and C2v isomers of carbon trioxide | |
| Names | |
|---|---|
| IUPAC proper name Dioxiran-three-one | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) |
|
| PubChem CID |
|
| InChI
| |
| SMILES
| |
| Properties | |
| Chemical formula | C O three |
| Molar mass | 60.008 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard land (at 25 °C [77 °F], 100 kPa). Infobox references | |
Carbon trioxide (COthree ) is an unstable oxide of carbon (an oxocarbon). The possible isomers of carbon trioxide include ones with molecular symmetry point groups Csouth , D3h , and C2v. The C2v state, consisting of a dioxirane, has been shown to be the basis state of the molecule.[ane] Carbon trioxide should non be confused with the stable carbonate ion (CO two−
3 ).
Carbon trioxide can be produced, for instance, in the drift zone of a negative corona discharge by reactions between carbon dioxide (CO2) and the diminutive oxygen (O) created from molecular oxygen by free electrons in the plasma.[ii] Another reported method is photolysis of ozone O3 dissolved in liquid CO2, or in COii/SFhalf-dozen mixtures at −45 °C, irradiated with light of 253.seven nm. The formation of COiii is inferred merely information technology appears to disuse spontaneously by the route
- two CO3 → ii CO2 + O2
with a lifetime much shorter than 1 minute.[3] Carbon trioxide tin can exist made by blowing ozone at dry ice (solid COii), and it has likewise been detected in reactions between carbon monoxide (CO) and molecular oxygen (O2). Along with the ground state C2v isomer,[4] the kickoff spectroscopic detection of the D3h isomer was in electron-irradiated ices of carbon dioxide.[5]
References [edit]
- ^ T. Kowalczyk; A. I. Krylov (Aug 2007). "Electronic structure of carbon trioxide and vibronic interactions involving Jahn–Teller states". J. Phys. Chem. A. 111 (33): 8271–8276. Bibcode:2007JPCA..111.8271K. doi:10.1021/jp073627d. ISSN 1089-5639. PMID 17661455.
- ^ Sabin, J. R; Kim, H (1971). "A theoretical report of the structure and properties of carbon trioxide". Chemical Physics Letters. 11 (5): 593–597. Bibcode:1971CPL....eleven..593S. doi:10.1016/0009-2614(71)87010-0.
- ^ DeMore W. B.; Jacobsen C. W. (1969). "Germination of carbon trioxide in the photolysis of ozone in liquid carbon dioxide". Journal of Physical Chemistry. 73 (9): 2935–2938. doi:ten.1021/j100843a026.
- ^ Bennett, Chris J.; Jamieson, C.; Mebel, Alexander M.; Kaiser, Ralf I. (2004). "Untangling the formation of the circadian carbon trioxide isomer in low temperature carbon dioxide ices". Physical Chemistry Chemical Physics. 6 (4): 735. Bibcode:2004PCCP....6..735B. doi:x.1039/b315626p. S2CID 51769127.
- ^ Jamieson, Corey S.; Mebel, Alexander Chiliad.; Kaiser, Ralf I. (2006). "Identification of the D3h Isomer of Carbon Trioxide (CO3) and Its Implications for Atmospheric Chemical science". ChemPhysChem. 7 (12): 2508–2513. doi:10.1002/cphc.200600390. PMID 17029325.
Further reading [edit]
- Sobek V.; Skalný J. D. (1993). "A simple model of processes in the drift region of negative corona discharge in a mixture of air with halocarbons". Czechoslovak Periodical of Physics. 43 (8): 807. Bibcode:1993CzJPh..43..807S. doi:10.1007/BF01589802. S2CID 120356317.
- Pople J. A.; Seeger U.; Seeger R.; Schleyer P. v. R. (2004). "The structure of carbonate". Journal of Computational Chemistry. 1 (two): 199–203. doi:x.1002/jcc.540010215. S2CID 98748631.
- Moll N. G.; Clutter D. R.; Thompson W. E. (1966). "Carbonate: Its Product, Infrared Spectrum, and Structure Studied in a Matrix of Solid CO2". The Journal of Chemic Physics. 45 (12): 4469–4481. Bibcode:1966JChPh..45.4469M. doi:ten.1063/ane.1727526.
- Gimarc B. M.; Chou T. South. (1968). "Geometry and Electronic Structure of Carbon Trioxide". The Journal of Chemical Physics. 49 (9): 4043–4047. Bibcode:1968JChPh..49.4043G. doi:10.1063/1.1670715.
- DeMore West. B.; Dede C. (1970). "Pressure dependence of carbon trioxide formation in the gas-stage reaction of O(1D) with carbon dioxide". Journal of Concrete Chemistry. 74 (13): 2621–2625. doi:10.1021/j100707a006.
- Francisco J. Due south.; Williams I. H. (1985). "A theoretical written report of the force field for carbon trioxide". Chemical Physics. 95 (3): 373. Bibcode:1985CP.....95..373F. doi:10.1016/0301-0104(85)80160-9.
Cr2 Co3 3 Compound Name,
Source: https://en.wikipedia.org/wiki/Carbon_trioxide
Posted by: mizellesompan.blogspot.com


0 Response to "Cr2 Co3 3 Compound Name"
Post a Comment